-

Genetics In Drug Discovery: The Key To Clinical Trial Success?
Article August 28, 2024
By leveraging human genetics to identify drug targets with a proven link to disease mechanisms, the probability of clinical trial success greatly increases [1]. Only 10% of drugs that enter phase 1 clinical trials eventually make it to market [2], primarily due to issues related to efficacy or safety that arise later on. These frequent […]
-
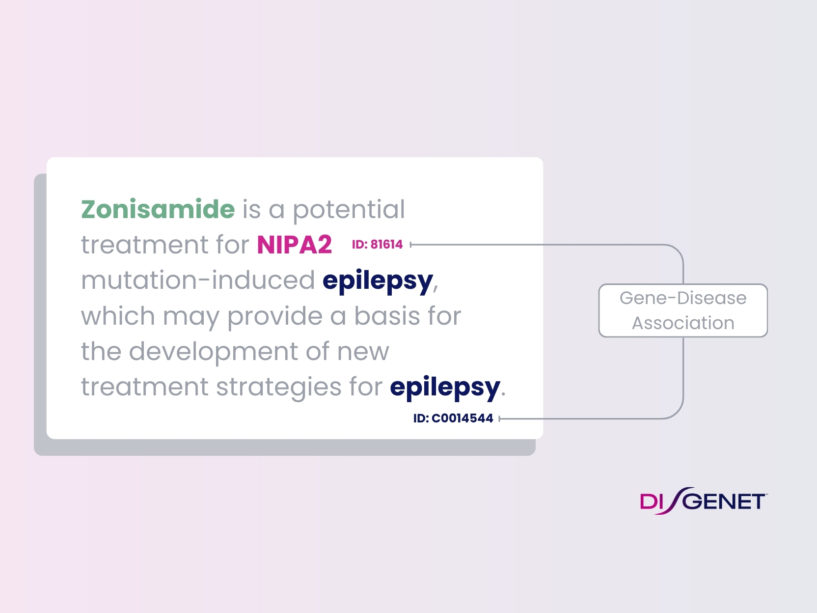
How DISGENET Uses NLP To Accelerate Drug Development
Article, Product July 19, 2024
With DISGENET, you can easily unlock new insights and greatly accelerate your drug development journey. Every year, more than 1 million papers enter PubMed in the biomedical field [1]. While medical knowledge doubles every 73 days and is increasing at an exponential rate with no evidence of slowing [2], drug discovery and development remain a […]
-

DISGENET Rebrand: A Fresh Look, Same Reliable Data for Tomorrow
News July 8, 2024
DISGENET — our gene-disease association knowledge platform — was created in 2010 driven by our mission that a greater understanding of human genetics was vital to improve people’s health. With over 100,000 web users, we see you agree. Today, that need for reliable, high-quality genomics data hasn’t faltered, but the way we access data has […]
-

DISGENET: The Next Chapter For Disease Genomics Research
Product June 19, 2024
From today, DisGeNET.org and DISGENET plus will become one unified platform found at disgenet.com. DisGeNET has become a cornerstone for bioinformatics research since starting in 2010. As a small team, we have dedicated 15 years to this valuable resource and we are committed to continuously improving the platform for you, our users. As you can imagine, […]
-
Anticipating Drug Toxicity: data-driven drug safety with DISGENET
Article September 12, 2023
Key points What is flupirtine? Flupirtine, an aminopyridine introduced in 1984 as a non-opioid analgesic for acute and chronic pain, embarked on a tumultuous journey within the medical landscape. Initially approved for broad pain management, its trajectory shifted in 2013 due to concerns over liver toxicity. The European Medicines Agency (EMA) limited its use to […]
-
Genetics for drug and chemical risk assessment
Article March 9, 2023
In recent years, there has been an increasing appreciation of the need to consider variability in human populations in evaluating health risks for new and existing chemicals. Recent policies such as The Frank R. Lautenberg Chemical Safety for the twenty-first Century Act (2016) require the US Environmental Protection Agency to evaluate new and existing toxic chemicals with explicit […]
-

Genetic support for FDA approvals in 2021
Article October 6, 2022
Key points Human genetics and FDA drug approvals in 2021 There is compelling evidence of the value of incorporating genetic information in the drug development process. A drug target supported by genetic evidence has a 2-fold higher probability of successful clinical development compared to those targets with no genetic support [1]. A systematic analysis of FDA […]
-

Meet our team: Jordi Valls
Interview May 6, 2022
Jordi Valls is a Postdoctoral Researcher at MedBioinformatics and experienced in the field of genomic analysis. Dr Valls is currently working on RISKHUNT3R, a European H2020 Project. He is involved in characterising and identifying the adverse effects of chemical compounds on cohorts/target segments depending on their genetic information and variability, through the use of different […]
